DRY CELL BATTERIES
In our world today, there is so much technology. Kids know how to do so much with technology stuff. But do they know what powers a lot of these things that make our life's so easy? Most of the new, hottest, coolest gadgets and cool tech stuff is powered by batteries of some sort. This page will talk about Dry cell batteries.
WHAT IS A DRY CELL BATTERY?
You have probably seen, used,or bought of a dry cell battery. These batteries are the ones that we use in remotes, some mp3 players, walkmans, cordless phones, some digital cameras, walky-talkies, and other electronics. The common dry cell battery is the zinc-carbon battery, using a cell sometimes called a dry Leclanche cell. This has the same voltage as a alkaline battery. Today most batteries are alkaline or lithium. Willie's definition of a dry cell battery is" a galvanic electrochemical cell with a pasty low-moisture electrolyte. I know, those are huge words. Allow me to break them down for you: a battery really is just a can or a small cylinder filled with chemicals and things that produce electrons.Have you ever heard a person referring to their battery " my juice is getting low"? Well, a dry cell does not have that juice, that moisture . However, while a dry cell's electrolyte is not all the way free of moisture it has has the advantage of not containing and liquid that could spill or leak when dropped or tipped. This is why some electronics are powered by dry cell batteries, so no "juice" can spill out. It is highly suitable for small portable electric devices.

above: Alkaline battery.
How does a dry cell battery work?
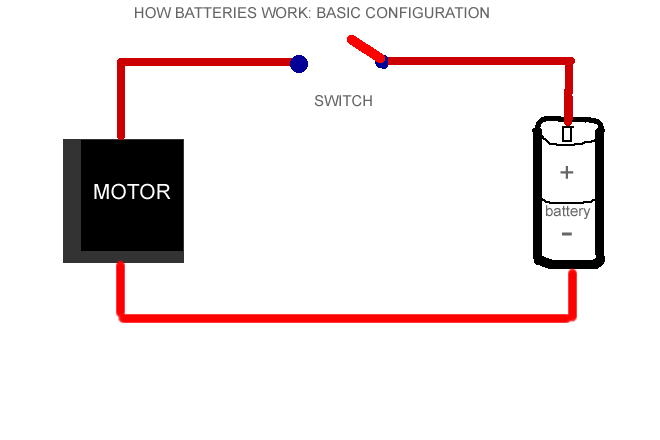
So previously stated, a battery is like a can filled with chemicals, right? Well, the chemicals that are in the can produce chemical reactions. Here is an important definition: Electrochemical reaction- chemical reactions that produce electrons.As you probably know, a battery has a plus sign(positive) or a negative sign(-). These are called terminals. Electrons gather on the negative terminal of the battery. Inside the battery, a chemical reaction produces the electrons. The battery's internally resistance( the speed of electron production by this chemical reaction) controls the number of electrons that can flow between terminals.Next the electrons flow from the battery into a wire. In order for the battery to work, they must travel from the negative to the positive terminal for the chemical reaction to take place. Unless the chemicals are flowing from the negative to the positive terminal, the chemical reaction does not take place.( This is why your mp3 player wont work when you put your batteries in wrong- after saying"why wont this stupid thing work- you eventually just realize that the batteries are in wrong - then you feel stupid. :)!!!)
COMMON DEVICES THAT USE DRY-CELL BATTERIES





So really, a battery is very useful in our technology world! Now you know how they work!

