Lithium Batteries
What is a lithium battery?
You have heard the saying "big things come in small packages" , right? Well that is the exact truth for lithium batteries. They are a really powerful , dense, energy sufficient battery all in a little, tiny package. This is why cell phones, laptops, some cameras, and other electronics run off lithium batteries-they are the ideal choice.
How does a lithium battery work?
The real name of a lithium battery is a lithium-ion battery. The name clues you in on how this thing works. Lithium batteries work by streaming lithium ions between the anode and cathode of the battery. Cathode-receptor of the ions and electrons. Anode- source of the ions and electron is elemental lithium( or a lithium-containing compound). These materials have the capability of receiving lithium ions into its structure. Populated by electrons,lithium ions stream from the anode to the cathode, when a battery is discharged. This stream of electrons is called the electrical current. The electronic current is what allows you to type that research paper on your laptop or to listen to Chris Brown on your Ipod, or even to text message your BFF Jill on your PDA. You charge your laptop, Ipod, PDA by supplying an external electric current ( a plug in the wall or other sources of energy) , which transports the lithium ions to return back to the anode. When you charge your laptop or whatever it may be, this process "restarts" the anode and cathode so that the battery can power your PDA again so that you can check your Myspace. So now you know how " cool" your PDA is- anything that small that can do all of this is pretty amazing!
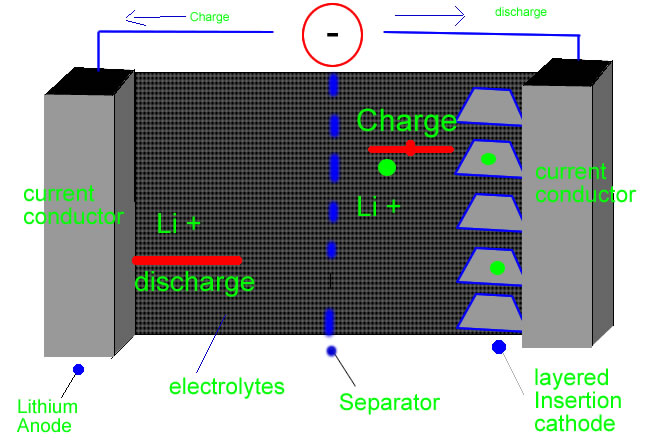
Advantages of using Lithium Batteries:
-
They are light
-
They are energetic-very high energy density .
-
They hold their charge.
-
They have no memory effect ( you don't have to completely discharge them before recharging)
-
They can take hundreds of charges/discharges- quality stuff-heavy, they are heavy duty
Disadvantages:
- They start to degrade the moment they leave the factory. They only last two to three years from the when the manufactures make them to whether you use them or not.
- They are very sensitive to high temps. If it is hotter, they degrade faster.
- If the lithium battery is completely discharged a lithium-ion battery, it ruined.
- They are expensive.
- Small percentage of catching fire.

